Clinical Trial Management Systems Market Projected To Grow USD 4.37 Billion By 2032
Global Clinical Trial Management System Market size is expected to be worth around USD 4.37 Bn by 2032 from USD 1.8 Bn in 2023, growing at a CAGR of 10.3%
NEW YORK CITY, NY, UNITED STATES, February 7, 2025 /EINPresswire.com/ -- Report Overview
New York, NY – February 07, 2025 –Global Clinical Trial Management System Market size is expected to be worth around USD 4.37 Bn by 2032 from USD 1.8 Bn in 2023, growing at a CAGR of 10.3% during the forecast period from 2023 to 2032.
The Clinical Trial Management System (CTMS) is becoming essential in clinical research, streamlining operations and improving efficiency in trial management. CTMS software assists pharmaceutical companies, research institutions, and contract research organizations (CROs) in managing clinical trials by tracking milestones, budgets, and regulatory compliance.
With the increasing complexity of clinical trials, the demand for CTMS is rising. The system enhances trial efficiency by automating patient enrollment, site monitoring, and data management. Advanced CTMS platforms integrate with electronic health records (EHRs) and regulatory databases, ensuring seamless data exchange and compliance with FDA and EMA guidelines.
The market is experiencing significant growth, driven by rising drug development activities, regulatory requirements, and increasing adoption of cloud-based solutions. Key players in the CTMS industry are focusing on AI-driven analytics, decentralized trial management, and enhanced security features. The expansion of CTMS is expected to accelerate due to increased investments in clinical research and personalized medicine. As the healthcare sector embraces digital transformation, CTMS will remain a vital tool in ensuring faster, more efficient, and compliant clinical trials.
Unlock Competitive Advantages With Our PDF Sample Report @ https://marketresearch.biz/report/clinical-trial-management-system-market/request-sample/
Key Takeaways
•Market Size: Clinical Trial Management System Market size is expected to be worth around USD 4.37 Bn by 2032 from USD 1.8 Bn in 2023.
•Market Growth: The market growing at a CAGR of 10.3% during the forecast period from 2023 to 2032.
•Enterprise CTMS Dominance – Large-scale pharmaceutical and biopharmaceutical companies prefer enterprise CTMS for managing multi-location trials and complex operations.
•Web-based CTMS Leads – Web-based solutions dominate due to remote accessibility, real-time data sharing, and scalability, especially in emerging economies.
•Pharmaceutical & Biotech Firms Drive Demand – These firms require CTMS to handle personalized medicine trials and precision therapies, ensuring compliance and efficiency.
•AI Integration is Transforming CTMS – AI-driven analytics enhance patient recruitment, data management, risk prediction, and decentralized trial operations.
•Regulatory Compliance is a Key Factor – CTMS adoption is increasing due to stringent regulations from FDA, EMA, and other authorities, ensuring trial transparency and efficiency.
•Decentralized Trials are Rising – Growing adoption of remote and virtual trials is fueling demand for digital CTMS platforms with advanced monitoring capabilities.
•Cost and Implementation Challenges – Despite growth, high costs and complexity in CTMS integration remain key barriers for smaller organizations.
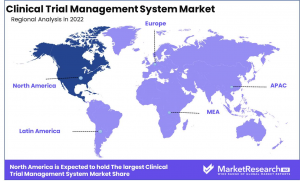
•North America Leads the Market – The region dominates due to advanced healthcare infrastructure, increased R&D investments, and high clinical trial activity.
How Artificial Intelligence (AI) is Transforming the Clinical Trial Management Systems Market ?
• Artificial Intelligence (AI) is revolutionizing the Clinical Trial Management Systems (CTMS) market by improving efficiency, accuracy, and decision-making. AI-powered CTMS platforms help streamline complex trial processes, enhance patient recruitment, and optimize trial monitoring.
• Faster Patient Recruitment & Screening: AI-driven algorithms analyze vast datasets to identify eligible participants based on medical history, genetic profiles, and real-world evidence. This accelerates recruitment, reducing delays and improving trial efficiency.
• Enhanced Trial Monitoring & Risk Prediction: AI-driven predictive analytics identify potential risks, such as patient dropouts and adverse events, allowing researchers to take preventive measures. Machine learning models analyze real-time data to ensure trials remain on track.
• Automated Data Management & Compliance: AI improves data accuracy by automating data collection, entry, and validation. Natural Language Processing (NLP) enables efficient handling of clinical documentation, ensuring compliance with regulatory standards such as FDA and EMA guidelines.
• Decentralized & Remote Trial Support: AI-driven chatbots and virtual assistants facilitate remote patient monitoring, improving engagement and adherence. AI also supports decentralized trials by enabling remote site management and real-time data processing.
• Optimized Trial Design & Drug Development: AI models analyze historical trial data and suggest optimal trial designs, reducing trial failures and expediting drug development. AI-powered simulations help in refining dosages and patient selection criteria.
Segmentation Analysis
By Type Analysis: The Enterprise CTMS Segment dominates the market due to its ability to handle large-scale operations and provide customizations for pharmaceutical and biopharmaceutical companies. These organizations conduct multi-location trials, requiring advanced monitoring and management features. With the increasing demand for personalized medicine and precision therapies, the segment is expected to grow rapidly, driven by its adaptability and ability to support complex clinical trial operations.
By Delivery Mode Analysis: The Web-based CTMS Segment leads the market due to its convenience, real-time data access, and remote collaboration features. This segment is crucial for clinical trials conducted in remote locations, especially in emerging economies. With rising demand for decentralized trials and increased reliance on digital solutions, web-based CTMS is expected to witness the highest growth rate, offering flexibility, scalability, and seamless trial management across multiple locations.
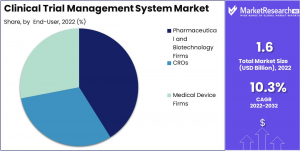
By End User Analysis: The Pharmaceutical and Biotechnology Firms Segment dominates the Clinical Trial Management System Market, requiring advanced solutions for multi-location trials and complex operations. The growing emphasis on personalized medicine and precision therapies drives demand for highly adaptable CTMS platforms. This segment is anticipated to grow significantly, as pharmaceutical and biotech companies increasingly adopt advanced CTMS solutions to streamline operations and enhance trial efficiency.
Market Segments
By Type
•Enterprise
•Site
By Delivery Mode
•Web-based
•Cloud-based
•On-premise
•By Component
Software
•Service
•By End-User
Pharmaceutical and Biotechnology Firms
•CROs
•Medical Device Firms
Buy This Premium Research Report: https://marketresearch.biz/purchase-report/?report_id=6457
Market Dynamics
• Driver: The increasing complexity of clinical trials necessitates advanced management solutions. As trials become more intricate, involving multiple sites and diverse data points, the demand for efficient Clinical Trial Management Systems (CTMS) has risen. These systems streamline trial processes, ensuring compliance with regulatory standards and enhancing data accuracy. The World Health Organization emphasizes the importance of well-implemented trials to address endemic conditions and respond effectively during health emergencies.
• Trend: There is a notable shift towards decentralized and remote clinical trials, facilitated by advancements in digital technologies. This trend allows for patient participation from various locations, reducing geographical barriers and enhancing recruitment. The European Medicines Agency has acknowledged the adoption of decentralized methodologies, which include remote monitoring and electronic data capture, to improve trial efficiency and patient engagement.
• Restraint: Despite technological advancements, the Clinical Trial Management System market faces challenges due to complex and fragmented regulatory frameworks. In regions like the European Economic Area, lengthy timelines for trial approvals and compliance checks have deterred pharmaceutical companies, leading to a decline in clinical trial activities. A report highlighted that Europe's share of global commercial clinical drug trials decreased from 22% in 2013 to 12% in 2023, partly due to these regulatory complexities.
• Opportunity: The growing emphasis on personalized medicine presents a significant opportunity for the CTMS market. Tailoring treatments to individual patient profiles requires sophisticated data management and analysis capabilities. CTMS platforms equipped with advanced analytics can facilitate the design and management of personalized treatment protocols, ensuring efficient data handling and regulatory compliance. The World Health Organization underscores the need for innovative research and development models to make medical innovations accessible globally.
Competitive Landscape
The rapid expansion of the Clinical Trial Management System (CTMS) market is driven by the increasing need for efficient clinical trial operations. The industry is witnessing continuous innovation from key players, leading to the development of advanced CTMS solutions that streamline study design, data management, patient enrollment, and regulatory compliance.
Leading pharmaceutical and biotechnology companies invest heavily in research and development to enhance CTMS capabilities. Market leaders include Oracle Corporation, Parexel International Corporation, Medidata Solutions, Inc., Bioclinica, Inc., and Merge Healthcare, Inc. These companies focus on improving clinical trial efficiency, ensuring compliance, and optimizing trial workflows.
Oracle Corporation stands out with its Oracle Health Sciences Clinical One platform, which integrates electronic data capture, trial management, and clinical data management into a unified solution. This innovation simplifies trial processes, enhancing overall efficiency and compliance.
Similarly, Parexel International Corporation plays a pivotal role in accelerating drug development and optimizing trial management. Its ClinPhone RTSM platform supports real-time patient randomization, drug administration, and supply management, ensuring smooth trial execution. By integrating essential trial functions, Parexel enhances the capabilities of clinical trial stakeholders, improving efficiency and expanding research opportunities.
Top Key Players
•Oracle Corporation
•International Business Machines Corporation
•MedNet Solutions, Inc.
•Wipro Limited
•Veeva Systems
•Bio-Optronics Inc.
•Cognizant Technology Solutions Corporations
•Medidata Solutions Inc.
•IQVIA Inc.
•DSG, Inc.
•Forte Research Systems, Inc.
Emerging Trends in Clinical Trial Management Systems
•Decentralized Clinical Trials (DCTs): Clinical trials are increasingly moving away from traditional centralized locations to decentralized models. This approach allows participants to engage from their homes or local healthcare facilities, enhancing accessibility and convenience. The U.S. Food and Drug Administration (FDA) acknowledges the growing role of DCTs and digital health technologies in modernizing clinical research.
•Integration of Artificial Intelligence (AI): AI and machine learning are being incorporated into clinical trial design and data analysis. These technologies assist in patient recruitment by analyzing large datasets to identify suitable candidates and predict outcomes, thereby improving trial efficiency. The FDA has noted the increasing use of AI in clinical research, particularly in trial design and real-world data analytics.
•Use of Digital Health Technologies (DHTs): Wearable devices and mobile health applications are being utilized to collect real-time data from participants. These tools monitor health metrics such as heart rate and activity levels, providing continuous data streams that enhance the understanding of treatment effects. The FDA has issued guidance on the use of DHTs for remote data acquisition in clinical investigations.
•Enhanced Data Management and Compliance: There is a growing emphasis on the use of computerized systems to manage clinical trial data. These systems ensure data integrity, security, and compliance with regulatory standards. The European Medicines Agency (EMA) has provided guidelines on the use of computerized systems and electronic data in clinical trials to assist sponsors and investigators in complying with regulatory requirements.
Use Cases of Clinical Trial Management Systems
•Patient Recruitment and Enrollment: CTMS platforms streamline the process of identifying and enrolling participants. By integrating with electronic health records (EHRs), these systems can match trial criteria with patient data, accelerating recruitment. For instance, a study demonstrated an approach to transmit structured data from an EHR system to a clinical trial electronic data capture system, facilitating efficient patient recruitment.
•Data Collection and Monitoring: CTMS platforms facilitate the collection and monitoring of trial data. By integrating with electronic health records (EHRs), these systems enable real-time data capture and remote monitoring, enhancing data accuracy and reducing the need for on-site visits. The FDA has provided guidance on the use of electronic source data in clinical investigations, highlighting the benefits of direct data capture from EHRs.
•Regulatory Compliance and Reporting: CTMS platforms assist in maintaining compliance with regulatory requirements by providing audit trails, electronic signatures, and secure data storage. They facilitate the generation of necessary documentation and reports for regulatory submissions. The FDA's guidance on computerized systems used in clinical trials outlines the expectations for system features to ensure compliance.
•Resource Management: CTMS platforms aid in managing resources by tracking site performance, monitoring patient enrollment, and managing study finances. This ensures efficient allocation of resources and timely completion of trial milestones. The FDA's risk-based approach to monitoring clinical investigations emphasizes the importance of efficient resource management to enhance trial quality.
Lawrence John
Prudour
+91 91308 55334
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release